-
- 当然,随着中国实力的增强,在这种"心理战"中获胜的几率也不是没有――奥巴马在成功会见胡锦涛之前,一直小心翼翼避免和达赖喇嘛见面。
- 这句有点讽刺了哈
-
2011年11月30日星期三
SocialPipeline 12/01/2011 (a.m.)
2011年11月29日星期二
2011年11月28日星期一
2011年11月26日星期六
SocialPipeline 11/26/2011 (p.m.)
-
1,阅读文字的感觉——超乎购买前所有相关(赞扬)信息的预期; 2,分类建立文件夹,如电子书/博客/收藏/推送文章等,纯属于个人习惯或喜好吧。 3,大赞推送功能,现在网上的高质量文章越来越多
1,阅读文字的感觉——超乎购买前所有相关(赞扬)信息的预期; 2,分类建立文件夹,如电子书/博客/收藏/推送文章等,纯属于个人习惯或喜好吧。 3,大赞推送功能,现在网上的高质量文章越来越多,而工作有效时间也越来越用不够,推送到KINDLE上阅读,妙不可言,大赞推送,大赞EINK技术; 4,以阅读MOBI格式的电子书为主; 5,主要通过CALIBRE和FREE.KINDLE.COM传书,偶尔要硬连接传送文件(如MP3)。 6,每周用KINDLE3听1小时左右音乐 最后,对KINDLE3的设计及制作工艺,也要赞上一赞,这是我的第一个EREADER,注定是最喜欢的一个,但绝不会是最后一个。
2011年11月24日星期四
2011年11月23日星期三
SocialPipeline 11/23/2011 (p.m.)
-
Buy the kindle 4(w/o keyboard, w/ ads) for one month, and reading <
> and some banned chinese books in china. Find here from a blog named "NTU" (http://startupno1.appspot.com/?p=5001) -
【分享】韩寒的《青春》,台湾版,一字未删。
-
我已经购买了一台kindle touch,尚未收到,因此严格来说不算有kindle阅读经验。说说纸书阅读经验吧。重要的是品质,一本校订精当、纸质良好、字体合适的书,可以让人非常投入地阅读,并且获取一种享受。我想,这一点到了kindle上面是不变的。我自己是一个喜欢读书的人,并且把读书作为主要的业余爱好,但读的还不够多
tags: kindler
2011年11月21日星期一
SocialPipeline 11/21/2011 (p.m.)
-
分享:郑念的"Life And Death in Shanghai"英文版
郑念的"Life And Death in Shanghai"英文版
-
tags: Visualization
-
tags: Visualization
-
BBC News - Eurozone debt web: Who owes what to whom?
tags: Visualization
-
- Twit 现场,这个节目名称和推特服务差不多,但是比推特时间更早,专门介绍各种科技动态,非常耐听,不知道Kin
dle Fire是否更有效集成这类媒体。Edit
-
-
喜歡英文、港台書籍,閱讀興趣廣泛。很喜歡kindle的閱讀體驗,正在讀的書有張大春的《城邦暴力團》、龍應台《大江大海1949》還有一些英文暢銷書。希望能加入組織和大家更好的分享kindle讀書體驗和心得:)
-
喜歡英文、港台書籍,閱讀興趣廣泛。很喜歡kindle的閱讀體驗,正在讀的書有張大春的《城邦暴力團》、龍應台《大江大海1949》還有一些英文暢銷書。希望能加入組織和大家更好的分享kindle讀書體驗和心得:)
tags: kindler
2011年11月20日星期日
SocialPipeline 11/20/2011 (p.m.)
-
Visualization: The Spread of Dance Music - Forbes
tags: Visualization
-
A new visualization by travel writer Osman Khan charts the “Evolution of Western Dance Music,” tracing music through time and space from Africa and the Caribbean through the development of the Blues, Jazz, Funk, Disco, and, well, you know the story.
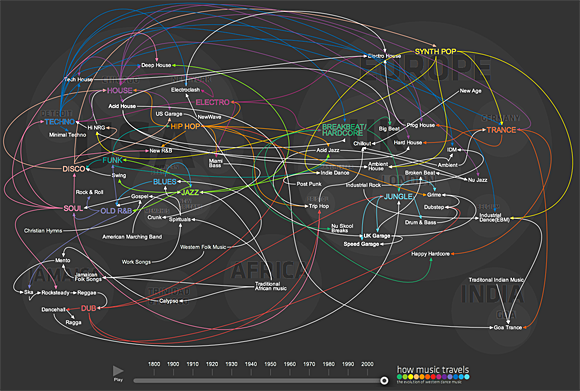
Screenshot of “The Evolution of Western Dance Music” visualization. Click here for the interactive version.Khan recognizes the spread of music is open to debate — in terms of how you define genre and influence, for example. (I’d also add, in terms of the size you choose to depict the continents in the associated visualization.) Khan points to his sources: Bass Culture, Last Night A DJ Saved My Life, and The All Music Guide to Electronica, along with Wikipedia.
The data marketplace Infochimps (where I found the link to Khan’s visualization) asks an interesting question: How would a visualization about the spread of music based on something like Infochimps’ Million Song dataset — a dataset about sound and recording metadata — differ from a visualization, like Khan’s, based on stories?
-
2011年11月19日星期六
SocialPipeline 11/19/2011 (p.m.)
-
One on One 用准确的1001个词讲述了101个六度关系的故事
用准确的1001个词讲述了101个六度关系的故事
- 用准确的1001个词讲述了101个六度关系的故事
-
-
我是名大学生,喜好阅读,无奈喜欢的书很多都被禁了。我是在twitter上知道这个网站的,我没有kindle的阅读经验,因为...买不起....
我是名大学生,喜好阅读,无奈喜欢的书很多都被禁了。我是在twitter上知道这个网站的,我没有kindle的阅读经验,因为...买不起....
tags: kindler
-
Nature and nurture work together to shape the brain | KurzweilAI
-
- Brain cell activation changes a protein involved in turning genes on and off, suggesting the protein may play a role in brain plasticity.
- Prenatal exposure to amphetamines and alcohol produces abnormal numbers of chromosomes in fetal mouse brains. The findings suggest these abnormal counts may contribute to the developmental defects seen in children exposed to drugs and alcohol in utero.
- Cocaine-induced changes in the brain may be inheritable. Sons of male rats exposed to cocaine are resistant to the rewarding effects of the drug.
- Motherhood protects female mice against some of the negative effects of stress.
- Mice conceived through breeding — but not those conceived through reproductive technologies — show anxiety-like and depressive-like behaviors similar to their fathers. The findings call into question how these behaviors are transmitted across generations.
At the Neuroscience 2011 conference, scientists at The Rockefeller University, The Scripps Research Institute, and the University of Pennsylvania presented new research demonstrating the impact that life experiences can have on genes and behavior. The studies examine how such environmental information can be transmitted from one generation to the next — a phenomenon known as epigenetics. This new knowledge could ultimately improve understanding of brain plasticity, the cognitive benefits of motherhood, and how a parent‘s exposure to drugs, alcohol, and stress can alter brain development and behavior in their offspring.
The new findings show that:
-
-
Batteries with 10x more capacity and 10x faster charge | KurzweilAI
" Clusters of silicon (green) between graphene sheets allow for more lithium atoms (blue) in electrodes for increased charging capacity and nanoholes speed up ion flow for faster charging speed (credit: Northwestern University)
Northwestern University engineers have created an electrode for lithium-ion batteries — rechargeable batteries such as those found in cellphones and iPods — that allows them to hold a charge up to 10 times greater and charge 10 times faster than current batteries; they could also pave the way for more efficient, smaller batteries for electric cars.
The technology could be seen in the marketplace in the next three to five years, the researchers said.
“We have found a way to extend a new lithium-ion battery’s charge life by 10 times,” said Harold H. Kung, professor of chemical and biological engineering.
“Even after 150 charges, which would be one year or more of operation, the battery is still five times more effective than lithium-ion batteries on the market today.”
How Lithium-ion batteries work
Lithium-ion batteries charge through a chemical reaction in which lithium ions are sent between two ends of the battery, the anode and the cathode. As energy in the battery is used, the lithium ions travel from the anode, through the electrolyte, and to the cathode; as the battery is recharged, they travel in the reverse direction.
With current technology, the performance of a lithium-ion battery is limited in two ways. Its energy capacity — how long a battery can maintain its charge — is limited by the charge density, or how many lithium ions can be packed into the anode or cathode. Meanwhile, a battery’s charge rate — the speed at which it recharges — is limited by another factor: the speed at which the lithium ions can make their way from the electrolyte into the anode.
In current rechargeable batteries, the anode — made of layer upon layer of carbon-based graphene sheets — can only accommodate one lithium atom for every six carbon atoms. To increase energy capacity, scientists have previously experimented with replacing the carbon with silicon, as silicon can accommodate much more lithium: four lithium atoms for every silicon atom. However, silicon expands and contracts dramatically in the charging process, causing fragmentation and losing its charge capacity rapidly.
Currently, the speed of a battery’s charge rate is hindered by the shape of the graphene sheets: they are extremely thin — just one carbon atom thick — but by comparison, very long. During the charging process, a lithium ion must travel all the way to the outer edges of the graphene sheet before entering and coming to rest between the sheets. And because it takes so long for lithium to travel to the middle of the graphene sheet, a sort of ionic traffic jam occurs around the edges of the material.
Battery charging breakthroughs
The research team has combined two techniques to combat both these problems. First, to stabilize the silicon in order to maintain maximum charge capacity, they sandwiched clusters of silicon between the graphene sheets. This allowed for a greater number of lithium atoms in the electrode while utilizing the flexibility of graphene sheets to accommodate the volume changes of silicon during use.
The team also used a chemical oxidation process to create miniscule holes (10 to 20 nanometers) in the graphene sheets — termed “in-plane defects” — so the lithium ions would have a “shortcut” into the anode and be stored there by reaction with silicon. This reduced the time it takes the battery to recharge by up to 10 times.
Next, the researchers will begin studying changes in the cathode that could further increase effectiveness of the batteries. They also will look into developing an electrolyte system that will allow the battery to automatically and reversibly shut off at high temperatures — a safety mechanism that could prove vital in electric car applications.
Ref.: Xin Zhao, et al., In-Plane Vacancy-Enabled High-Power Si–Graphene Composite Electrode for Lithium-Ion Batteries, Advanced Energy Materials, 2011; [DOI: 10.1002/aenm.201100426]"-
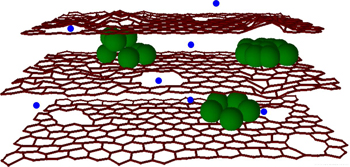
Clusters of silicon (green) between graphene sheets allow for more lithium atoms (blue) in electrodes for increased charging capacity and nanoholes speed up ion flow for faster charging speed (credit: Northwestern University)
Northwestern University engineers have created an electrode for lithium-ion batteries — rechargeable batteries such as those found in cellphones and iPods — that allows them to hold a charge up to 10 times greater and charge 10 times faster than current batteries; they could also pave the way for more efficient, smaller batteries for electric cars.
The technology could be seen in the marketplace in the next three to five years, the researchers said.
“We have found a way to extend a new lithium-ion battery’s charge life by 10 times,” said Harold H. Kung, professor of chemical and biological engineering.
“Even after 150 charges, which would be one year or more of operation, the battery is still five times more effective than lithium-ion batteries on the market today.”
How Lithium-ion batteries work
Lithium-ion batteries charge through a chemical reaction in which lithium ions are sent between two ends of the battery, the anode and the cathode. As energy in the battery is used, the lithium ions travel from the anode, through the electrolyte, and to the cathode; as the battery is recharged, they travel in the reverse direction.
With current technology, the performance of a lithium-ion battery is limited in two ways. Its energy capacity — how long a battery can maintain its charge — is limited by the charge density, or how many lithium ions can be packed into the anode or cathode. Meanwhile, a battery’s charge rate — the speed at which it recharges — is limited by another factor: the speed at which the lithium ions can make their way from the electrolyte into the anode.
In current rechargeable batteries, the anode — made of layer upon layer of carbon-based graphene sheets — can only accommodate one lithium atom for every six carbon atoms. To increase energy capacity, scientists have previously experimented with replacing the carbon with silicon, as silicon can accommodate much more lithium: four lithium atoms for every silicon atom. However, silicon expands and contracts dramatically in the charging process, causing fragmentation and losing its charge capacity rapidly.
Currently, the speed of a battery’s charge rate is hindered by the shape of the graphene sheets: they are extremely thin — just one carbon atom thick — but by comparison, very long. During the charging process, a lithium ion must travel all the way to the outer edges of the graphene sheet before entering and coming to rest between the sheets. And because it takes so long for lithium to travel to the middle of the graphene sheet, a sort of ionic traffic jam occurs around the edges of the material.
Battery charging breakthroughs
The research team has combined two techniques to combat both these problems. First, to stabilize the silicon in order to maintain maximum charge capacity, they sandwiched clusters of silicon between the graphene sheets. This allowed for a greater number of lithium atoms in the electrode while utilizing the flexibility of graphene sheets to accommodate the volume changes of silicon during use.
The team also used a chemical oxidation process to create miniscule holes (10 to 20 nanometers) in the graphene sheets — termed “in-plane defects” — so the lithium ions would have a “shortcut” into the anode and be stored there by reaction with silicon. This reduced the time it takes the battery to recharge by up to 10 times.
Next, the researchers will begin studying changes in the cathode that could further increase effectiveness of the batteries. They also will look into developing an electrolyte system that will allow the battery to automatically and reversibly shut off at high temperatures — a safety mechanism that could prove vital in electric car applications.
Ref.: Xin Zhao, et al., In-Plane Vacancy-Enabled High-Power Si–Graphene Composite Electrode for Lithium-Ion Batteries, Advanced Energy Materials, 2011; [DOI: 10.1002/aenm.201100426]
-
-
我是名大学生,喜好阅读,无奈喜欢的书很多都被禁了。我是在twitter上知道这个网站的,我没有kindle的阅读经验,因为...买不起....
我是名大学生,喜好阅读,无奈喜欢的书很多都被禁了。我是在twitter上知道这个网站的,我没有kindle的阅读经验,因为...买不起....
tags: kindler
-
- 射雕英雄传英文版
-
-
- 余华《十个词汇里的中国》
- K4比K3确实小不少,轻不少,但是电池容量差一半…K4外出时候可以放在屁股口袋里,很方便
- 冯唐的《不二》
-
-
Followers of my blog probably know that this blog is photo heavy. I get many compliments for posting them but I wanted to expand into posting my thoughts and observations on design and the industry. Of course, they will be complimented with graphics and photos.
I'll be calling these Coffee Time.
I'll post on a frequent basis. Most of them will be short (hence the name). This week, I wanted to share my thoughts on market share of iOS.
These numbers have been creating quite a buzz recently. Most people that readers have probably seen this already. The issue is that Android is dominating in terms of Android and people are having questions about how successful iOS really is.
What many people have failed to associate is that Apple dominates in terms of revenue. Look at these graphs by Oppenheimer. I've always categorized Android users into two key segments; nerds and budget buyers. The sad thing is that nerds are the minority. Most people are looking for the cheapest smartphone to purchase - and Android offers many of these choices. This is most likely the reason Android ships so many devices yet makes little margin. Also keep in mind that these graphs include Windows Phones too. There are many ways you can interpret these statistics. The key facts:
- Yes, in terms of a pure number of shipped products, Android rules all.
- If you look at the amount of money being made, Apple dominates.
- Android has many manufacturers using their OS, iOS devices are only made by Apple.
This is how I like to illustrate this whole scenario. Above is Apple's product line. Lean and simple.
These are the phones Samsung currently sells in the US. You be the judge. Samsung is only one of the many manufacturers selling Android phones and has this complex of a product lineup. And yes, there are non-Android phones in the mix but it shows how many phones other companies make only to make a fraction of what Apple does.
tags: Visualization
-
E-Reader Display Shows Vibrant Color Video - Technology Review
-
Even as the processing power and download speeds of mobile devices surge, one component still lags behind: the screen. LCD panels use significantly more power than any other component of a phone or tablet because of their need to pump out bright light to form an image.
The only practical alternative is e-ink, the technology used in the Amazon Kindle; it consumes orders of magnitude less power but sacrifices color and the ability to change images fast enough for video playback or smooth game play.
Now, after years of waiting, alternative technology that promises the best of both approaches is finally edging closer to commercialization. During a recent visit to mobile chipmaker Qualcomm's headquarters in San Diego, Technology Review tried out a full-color, 5.7-inch Android tablet with a display that offers rich colors under bright light, close to those of an LCD and not unlike the pages of a magazine. The prototype screen was also responsive enough for video playback and for a game of Angry Birds; it can deliver up to 30 frames per second.
Because the device's screen uses ambient light, like a printed page or e-ink display, the power consumption is a tenth or less of that of a comparable LCD, although the display also features a built-in light for use in the dark. Known as Mirasol, the technology was created by a startup company, Iridigm, acquired by Qualcomm in 2004.
<!--<p style="clear: both; text-align: center; font-size: 11px; padding:0; margin:0"><a href="#afteradbody" style="text-decoration: none;">Story continues below</a></p>-->
<script type="text/javascript"> <!--//--><![CDATA[//><!-- document.write('<SCR' + 'IPT LANGUAGE="JavaScript" SRC="//ad.doubleclick.net/adj/mk3.technologyreview.com/mediumrectangle4;!category=computingexc;channel=computing;section=;at=mediumrectangle4;page=home;s=mediumrectangle4;dcopt=ist;ord=' + randartnum + '?"><\/SCR' + 'IPT>') //--><!]> </script><script src="//ad.doubleclick.net/adj/mk3.technologyreview.com/mediumrectangle4;%21category=computingexc;channel=computing;section=;at=mediumrectangle4;page=home;s=mediumrectangle4;dcopt=ist;ord=3721448310665727?" language="JavaScript"></script>
"In the market today, you have the iPad at one end and things like e-ink at the other end. This is really meant to bridge both of those worlds," says Clarence Chui, who leads the group at Qualcomm developing the new technology. "It is extremely low power, full color, and can be looked at wherever you go."
The Mirasol display makes color in the same way as the wings of iridescent butterflies or peacock feathers—by being an imperfect mirror that tunes the color of incoming light before reflecting it back to the viewer.
In a Mirasol display, this is done by small cavities known as interferometric modulators, tens of microns across and a few hundred nanometers deep, beneath the display's glass surface. "It's the air gap between the back of that glass and a mirror membrane at the bottom of the modulator that sets the color," says Chui. Each modulator's mirror membrane can snap upward against the glass when a small voltage is applied, closing the cavity and displaying a black color to the viewer. Mirasol modulators are made using techniques similar to those used to pattern metals and deposit materials in computer chip manufacturing.
-
2011年11月18日星期五
SocialPipeline 11/19/2011 (a.m.)
-
republic wireless - what it costs
-
The republic philosophy. Down with phone bills, up with freedom.
Did you know that on an average mobile service bill, two-thirds of the cost comes from talking? Just talking! We think that’s screwy. That’s why we introduced Hybrid Calling. You pay $199 for the first month, plus tax, and get a new LG Optimus smartphone.
Not satisfied with the phone or service? Return it anytime in the first 30 days and receive your money back, no questions asked. Membership after that is $19 per month, plus tax. Period. Going Wi-Fi allows us to broom the whole business of contracts, overages and having any calling plan at all. You use your phone all you want. You say “so long” any time you want. republic wireless means freedom. Experience it sometime.
-
SocialPipeline 11/18/2011 (p.m.)
-
One Per Cent: Occupy vs Tea Party: what their Twitter networks reveal
tags: Visualization
- The equivalent Tea Party network for 15 November, analysing a similar number of tweets posted over about three hours, looks rather different:

(Image: Marc Smith of the Social Media Research Foundation)
Here there are two main clusters, the blue one on the left containing Republican presidential candidate Ron Paul and his supporters. (The diffuse cluster in the lower centre of the diagram consists not of Tea Party supporters, but critics of the movement from the political left.)
-
2011年11月17日星期四
2011年11月10日星期四
2011年11月6日星期日
SocialPipeline 11/06/2011 (p.m.)
-
Wolfram Demonstrations Project: Browse by Topic
-
mputational Geometry (view all »)
 Computer Algebra (view all »)
Computer Algebra (view all ») Computer Graphics (view all »)
Computer Graphics (view all ») Cryptography (view all »)
Cryptography (view all ») Data Compression (view all »)
Data Compression (view all ») Image Processing (view all »)
Image Processing (view all ») Numerical Analysis (view all »)
Numerical Analysis (view all ») Computer Science (view all »)
Computer Science (view all ») High School Computer Science (view all »)
High School Computer Science (view all ») Computational Geometry (view all »)
Computational Geometry (view all ») Computer Graphics (view all »)
Computer Graphics (view all ») Data Structures (view all »)
Data Structures (view all ») Finite State Machines (view all »)
Finite State Machines (view all ») Recursion (view all »)
Recursion (view all ») Theory of Computation (view all »)
Theory of Computation (view all ») Computer Systems
Computer Systems
2011年11月2日星期三
SocialPipeline 11/03/2011 (a.m.)
-
BBC中文网 - 科技健康 - 科学家发现“最古老欧洲人”遗骨
-
科学家说,这项研究还显示,四万多年前,智慧人类曾经在欧洲与尼安德塔人同时并存了三到五千年,也许对尼安德塔人的灭绝产生了重要影响。
-
SocialPipeline 11/02/2011 (p.m.)
-
fbKindle: 在 Kindle 上阅读 ePub 电子书 — Padevices
-
- Kindle 需要被越狱
- Kindle 上要安装有 Launchpad
- 从 MobileRead 下载 kindle-jailbreak-0.6.N.zip 越狱文件,并解包备用。
将 Kindle 连接至电脑,拷贝 update_jailbreak_0.6.N_xxx_install.bin 文件到 Kindle 根目录。其中,xxx 代表:
- k2:Kindle 2 US
- k2i:Kindle 2 国际版
- dx:Kindle DX US
- dxi:Kindle DX 国际版
- dxg:Kindle DX Graphite
- k3g:Kindle 3 3G US
- k3w:Kindle 3 WiFi
- k3gb:Kindle 3 3G UK
安全拔掉 Kindle,从 Home 页选择 Menu → Settings → Menu → Update Your Kindle 菜单项目来更新 Kindle。
Kindle 将予以提示,确认后系统开始更新。完成后 Kindle 将自动重启。
- 下载 lpad-pkg-001c.zip 文件,同样解包备用。
- 连接 Kindle 到电脑,将 update_launchpad_0.0.1c_xxx_install.bin 拷贝到 Kindle 根目录。关于 xxx 的解释同上。
- 最后一步,与 Kindle 越狱一样,通过 Update Your Kindle 菜单来更新 Kindle。完成后,Kindle 会重启。
- 下载 fbKindle-bin.tar.gz 文件,不用解包。
- 将 Kindle 与电脑相连,并把下载的文件拷贝到 Kindle 根目录。
转到 Kindle 根下的 launchpad 目录,打开其中的 launchpad.ini 文件,并添加下列内容:
;; run experimental FBReader for kindle F R = !/mnt/us/fbKindle/goqt.sh FBReader & ;; unpack experimental fbreader's tar archive U T = !cd /mnt/us; tar zxvf fbKindle-bin.tar.gz; rm fbKindle-bin.tar.gz; echo 101 >/proc/eink_fb/update_display断开 Kindle 与电脑的连接,并重启 Kindle。
- 在 Kindle 的 Home 页按 Shift+U+T(先按 Shift 键,接着是 U 和 T 键),此时 Kindle 的屏幕底部将显示
^[U T]字样。稍等一会儿,fbKindle 解压完成后,你将会看到 Kindle 变成灰屏。 至此,fbKindle 安装成功。按 Home 返回 Home 页。 - 现在按 Shift+F+R 就可以启动 fbKindle 了。如果要从 fbKindle 切换回 Kindle,按 Alt+Shift;反之也一样。 退出 fbKindle,则按 Alt+Back。
{ via MobileRead }

众所周知,Kindle 不支持阅读 ePub 格式的电子书。不过,利用 fbKindle 这款软件,我们可以去掉 Kindle 的限制,在 Kindle 上也可痛快阅读 ePub 电子书。
要在 Kindle 上安装 fbKindle,必须满足以下两个条件:
-